Learning Outcomes
Name: Oxygen Symbol: O Atomic Number: 8 Atomic Mass: 15.9994 amu Melting Point:-218.4 °C (54.750008 K, -361.12 °F) Boiling Point:-183.0 °C (90.15 K, -297.4 °F) Number of Protons/Electrons: 8 Number of Neutrons: 8 Classification: Non-metal Crystal Structure: Cubic Density @ 293 K: 1.429 g/cm 3 Color: colorless Atomic Structure. The atomic number of oxygen is 8 and the atomic mass of one isotope of oxygen is 17. How many neutrons does this isotope contain? The atomic number of oxygen is 8 and the atomic mass of one isotope of oxygen is 17. Chemistry Matter Atomic Number. 1 Answer anor277 Sep 13, 2016 Is it #8#? Explanation: How did I know that #Z=8#? Do I have the atomic numbers memorized?
- Define atomic and mass numbers.
- Determine the number of protons, neutrons, and electrons in an atom.
- Identify the charge and relative mass of subatomic particles.
- Label the location of subatomic particles in the atom.
- Determine the mass of an atom based on its subatomic particles.
- Write A/Z and symbol-mass format for an atom.
Atoms are the fundamental building blocks of all matter and are composed of protons, neutrons, and electrons. Because atoms are electrically neutral, the number of positively charged protons must be equal to the number of negatively charged electrons. Since neutrons do not affect the charge, the number of neutrons is not dependent on the number of protons and will vary even among atoms of the same element.

Atomic Number
The atomic number (represented by the letter Z)of an element is the number of protons in the nucleus of each atom of that element. An atom can be classified as a particular element based solely on its atomic number. For example, any atom with an atomic number of 8 (its nucleus contains 8 protons) is an oxygen atom, and any atom with a different number of protons would be a different element. The periodic table (see figure below) displays all of the known elements and is arranged in order of increasing atomic number. In this table, an element's atomic number is indicated above the elemental symbol. Hydrogen, at the upper left of the table, has an atomic number of 1. Every hydrogen atom has one proton in its nucleus. Next on the table is helium, whose atoms have two protons in the nucleus. Lithium atoms have three protons, beryllium atoms have four, and so on.
Since atoms are neutral, the number of electrons in an atom is equal to the number of protons. Hydrogen atoms all have one electron occupying the space outside of the nucleus. Helium, with two protons, will have two electrons. In the chemical classroom, the proton count will always be equivalent to an atom's atomic number. This value will not change unless the nucleus decays or is bombarded (nuclear physics).
Mass Number
Experimental data showed that the vast majority of the mass of an atom is concentrated in its nucleus, which is composed of protons and neutrons. The mass number (represented by the letter A)is defined as the total number of protons and neutrons in an atom. Consider the table below, which shows data from the first six elements of the periodic table.
| Name | Symbol | Atomic Number (Z) | Protons | Neutrons | Electrons | Mass Number (A) (rounded to two decimals) |
|---|---|---|---|---|---|---|
| hydrogen | (ce{H}) | 1 | 1 | 0 | 1 | 1.01 |
| helium | (ce{He}) | 2 | 2 | 2 | 2 | 4.00 |
| lithium | (ce{Li}) | 3 | 3 | 4 | 3 | 6.94 |
| beryllium | (ce{Be}) | 4 | 4 | 5 | 4 | 9.01 |
| boron | (ce{B}) | 5 | 5 | 6 | 5 | 10.18 |
| carbon | (ce{C}) | 6 | 6 | 6 | 6 | 12.01 |
Consider the element helium. Its atomic number is 2, so it has two protons in its nucleus. Its nucleus also contains two neutrons. Since (2 + 2 = 4), we know that the mass number of the helium atom is 4. Finally, the helium atom also contains two electrons, since the number of electrons must equal the number of protons. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. Lithium, for example, has three protons and four neutrons, giving it a mass number of 7.
Knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction.
[text{Number of neutrons} = text{ rounded mass number} - text{atomic number}]
Atoms of the element chromium (left( ce{Cr} right)) have an atomic number of 24 and a mass number of 52. How many neutrons are in the nucleus of a chromium atom? To determine this, you would subtract as shown:

[52 - 24 = 28 : text{neutrons in a chromium atom}]
The composition of any atom can be illustrated with a shorthand notation called A/Z format. Both the atomic number and mass are written to the left of the chemical symbol. The 'A' value is written as a superscript while the 'Z' value is written as a subscript. For an example of this notation, look to the chromium atom shown below:
[ce{^{52}_{24}Cr}]
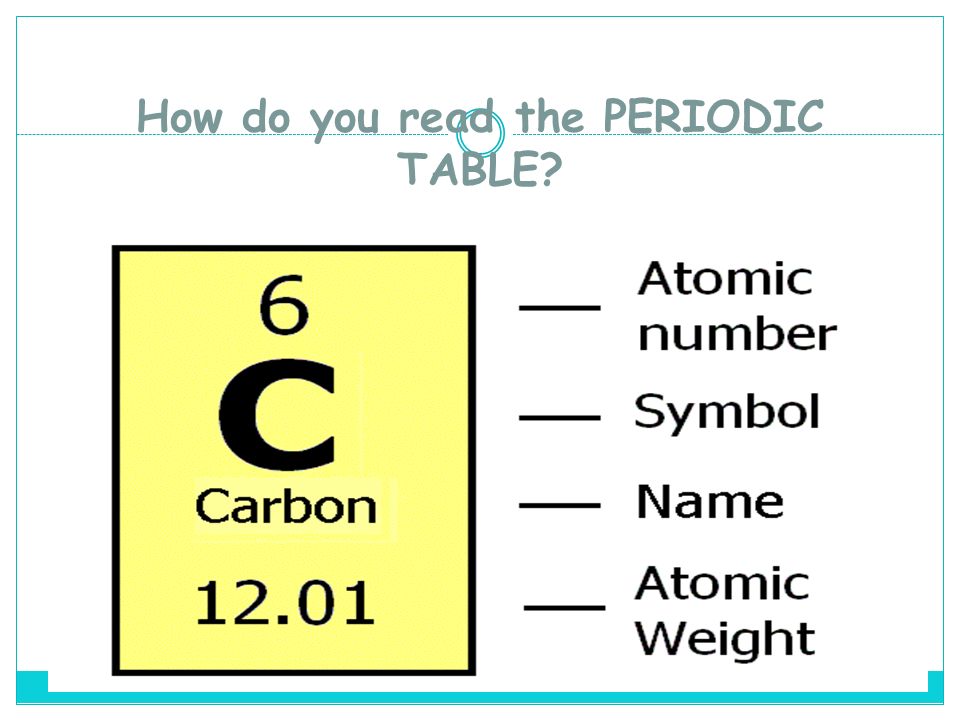
Another way to refer to a specific atom is to write the mass number of the atom after the name, separated by a hyphen. Symbol-mass format for the above atom would be written as Cr-52. In this notation, the atomic number is not included. You will need to refer to a periodic table for proton values.
Example (PageIndex{1})
Calculate each of the three subatomic particles and give specific group or period names for each atom.
- mercury
- platinum
- bromine
Solutions
- Hg (transition metal)- has 80 electrons, 80 protons, and 121 neutrons
- Pt (transition metal)- has 78 electrons, 78 protons, and 117 neutrons
- Br (halogen)- has 35 electrons, 35 protons, and 45 neutrons
Example (PageIndex{2})
Write both A/Z and symbol-mass formats for the atoms in Example (PageIndex{1}).
Solutions
- (ce{^{201}_{80}Hg}) and Hg-201
- (ce{^{195}_{78}Pt}) and Pt-195
- (ce{^{80}_{35}Br}) and Br-80
Example (PageIndex{3})
Identify the elements based on the statements below.
- Which element has 25 protons?
- Which element has 0 neutrons?
- Which element has 83 electrons?
Solutions
a. manganese
b. hydrogen
c. bismuth
Need More Practice?
- Turn to section 3.E of this OER and answer questions #1-#2, #4, and #8.
Contributors and Attributions
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.
Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky)
Atoms and Elements, Isotopes and Ions
Atoms are composed of protons,neutrons, andelectrons. A proton has an electric charge of +1 and a rest mass of 1.67 x 10-24 gm. A neutron has a charge of 0 and a rest mass of 1.67 x 10-24 gm. (about the same as a proton).An electron has a charge of -1 and a rest mass of 9.11 x 10-28 gm.(much, much less than a proton).The important point here is that the electron mass is negligible relative toprotons and neutrons.
The heavy particles (protons and neutrons) are bound into the nucleus, whereas the electrons form complex orbitals about the nucleus.
The chemical properties of an element depend on the number of protons (i.e. the net electric charge) of the nucleus. The number of protons in the nucleus is known as the atomic number of the element. Atomic numbers for natural element range from 1 (hydrogen) to 92 for uranium.
How To Find Atomic Number
The number of protons plus neutrons in the nucleus is known as the mass number of the atom. Atoms of a given element (atomic number) may have differing numbers of neutrons. Atoms of the same element with different mass numbers are known as isotopes. The mass numbers or isotopes of an element are denoted as preceding superscripts. For example the stable isotopes of the element oxygen are denoted 18O, 17O and 16O. Oxygen has an atomic number of 8 (eight protons). The nucleus of 16O thus contains eight protons and eight neutrons. How many neutrons are in the nucleus of 18O? (ans.: 10). Because elements may have several stable isotopes, the average mass number of an element is the atomic weight and is commonly not an integer.
Atomic Number Of Oxy
Atoms may not change their atomic numbers or mass numbers except by veryenergetic nuclear reactions. However atoms may gain or lose electrons in ordinary chemical reactions. If an atom has the same number of electrons as protons, it is a neutral atom. If it has a net charge, (more or less electrons than protons) it is an ion.If it has more electrons than protons it has a net negative charge and is known as an anion. If it has fewer electrons than protons it has a net positive charge and is known as a cation. The ionic state may be denoted as a following superscript (e.g. O2-, Fe2+). The common ionic states of a atom are known as its valences.
Because electrons arrange themselves in discreet orbitals about the nucleus and the orbitals repeat in shells, the chemical properties of the elements tend to repeat as the atomic number increases. This periodicity of properties gives rise to the periodic table of the elements.
Atomic Number Of O
The elements H, He, and minor amounts of Li were formed in the original Big Bang. All heavier elements were formed form the primordial H and He by nuclear fusion reactions in stars. The fusion reaction proceeds in steps in stars massive enough to undergo the full sequence. (Our sun is not massive enough to form elements more massive than He by direct fusion and will die when all the H is consumed.) First H is consumed to form He. When the H is consumed, the star collapses until He is 'ignited' to form Be and C. There are many free neutrons in these reactors and nuclei will capture enough neutrons to stabilize themselves. Most of the heavy elements are formed by neutron capture rather than by direct fusion. In the last stage Fe is formed by direct fusion of Si and other light elements. This reactions is rapid and results in an explosion. Our solar system condensed from the remnants of one of these supernova explosions.

Atomic Number
The atomic number (represented by the letter Z)of an element is the number of protons in the nucleus of each atom of that element. An atom can be classified as a particular element based solely on its atomic number. For example, any atom with an atomic number of 8 (its nucleus contains 8 protons) is an oxygen atom, and any atom with a different number of protons would be a different element. The periodic table (see figure below) displays all of the known elements and is arranged in order of increasing atomic number. In this table, an element's atomic number is indicated above the elemental symbol. Hydrogen, at the upper left of the table, has an atomic number of 1. Every hydrogen atom has one proton in its nucleus. Next on the table is helium, whose atoms have two protons in the nucleus. Lithium atoms have three protons, beryllium atoms have four, and so on.
Since atoms are neutral, the number of electrons in an atom is equal to the number of protons. Hydrogen atoms all have one electron occupying the space outside of the nucleus. Helium, with two protons, will have two electrons. In the chemical classroom, the proton count will always be equivalent to an atom's atomic number. This value will not change unless the nucleus decays or is bombarded (nuclear physics).
Mass Number
Experimental data showed that the vast majority of the mass of an atom is concentrated in its nucleus, which is composed of protons and neutrons. The mass number (represented by the letter A)is defined as the total number of protons and neutrons in an atom. Consider the table below, which shows data from the first six elements of the periodic table.
| Name | Symbol | Atomic Number (Z) | Protons | Neutrons | Electrons | Mass Number (A) (rounded to two decimals) |
|---|---|---|---|---|---|---|
| hydrogen | (ce{H}) | 1 | 1 | 0 | 1 | 1.01 |
| helium | (ce{He}) | 2 | 2 | 2 | 2 | 4.00 |
| lithium | (ce{Li}) | 3 | 3 | 4 | 3 | 6.94 |
| beryllium | (ce{Be}) | 4 | 4 | 5 | 4 | 9.01 |
| boron | (ce{B}) | 5 | 5 | 6 | 5 | 10.18 |
| carbon | (ce{C}) | 6 | 6 | 6 | 6 | 12.01 |
Consider the element helium. Its atomic number is 2, so it has two protons in its nucleus. Its nucleus also contains two neutrons. Since (2 + 2 = 4), we know that the mass number of the helium atom is 4. Finally, the helium atom also contains two electrons, since the number of electrons must equal the number of protons. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. Lithium, for example, has three protons and four neutrons, giving it a mass number of 7.
Knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction.
[text{Number of neutrons} = text{ rounded mass number} - text{atomic number}]
Atoms of the element chromium (left( ce{Cr} right)) have an atomic number of 24 and a mass number of 52. How many neutrons are in the nucleus of a chromium atom? To determine this, you would subtract as shown:
[52 - 24 = 28 : text{neutrons in a chromium atom}]
The composition of any atom can be illustrated with a shorthand notation called A/Z format. Both the atomic number and mass are written to the left of the chemical symbol. The 'A' value is written as a superscript while the 'Z' value is written as a subscript. For an example of this notation, look to the chromium atom shown below:
[ce{^{52}_{24}Cr}]
Another way to refer to a specific atom is to write the mass number of the atom after the name, separated by a hyphen. Symbol-mass format for the above atom would be written as Cr-52. In this notation, the atomic number is not included. You will need to refer to a periodic table for proton values.
Example (PageIndex{1})
Calculate each of the three subatomic particles and give specific group or period names for each atom.
- mercury
- platinum
- bromine
Solutions
- Hg (transition metal)- has 80 electrons, 80 protons, and 121 neutrons
- Pt (transition metal)- has 78 electrons, 78 protons, and 117 neutrons
- Br (halogen)- has 35 electrons, 35 protons, and 45 neutrons
Example (PageIndex{2})
Write both A/Z and symbol-mass formats for the atoms in Example (PageIndex{1}).
Solutions
- (ce{^{201}_{80}Hg}) and Hg-201
- (ce{^{195}_{78}Pt}) and Pt-195
- (ce{^{80}_{35}Br}) and Br-80
Example (PageIndex{3})
Identify the elements based on the statements below.
- Which element has 25 protons?
- Which element has 0 neutrons?
- Which element has 83 electrons?
Solutions
a. manganese
b. hydrogen
c. bismuth
Need More Practice?
- Turn to section 3.E of this OER and answer questions #1-#2, #4, and #8.
Contributors and Attributions
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.
Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky)
Atoms and Elements, Isotopes and Ions
Atoms are composed of protons,neutrons, andelectrons. A proton has an electric charge of +1 and a rest mass of 1.67 x 10-24 gm. A neutron has a charge of 0 and a rest mass of 1.67 x 10-24 gm. (about the same as a proton).An electron has a charge of -1 and a rest mass of 9.11 x 10-28 gm.(much, much less than a proton).The important point here is that the electron mass is negligible relative toprotons and neutrons.
The heavy particles (protons and neutrons) are bound into the nucleus, whereas the electrons form complex orbitals about the nucleus.
The chemical properties of an element depend on the number of protons (i.e. the net electric charge) of the nucleus. The number of protons in the nucleus is known as the atomic number of the element. Atomic numbers for natural element range from 1 (hydrogen) to 92 for uranium.
How To Find Atomic Number
The number of protons plus neutrons in the nucleus is known as the mass number of the atom. Atoms of a given element (atomic number) may have differing numbers of neutrons. Atoms of the same element with different mass numbers are known as isotopes. The mass numbers or isotopes of an element are denoted as preceding superscripts. For example the stable isotopes of the element oxygen are denoted 18O, 17O and 16O. Oxygen has an atomic number of 8 (eight protons). The nucleus of 16O thus contains eight protons and eight neutrons. How many neutrons are in the nucleus of 18O? (ans.: 10). Because elements may have several stable isotopes, the average mass number of an element is the atomic weight and is commonly not an integer.
Atomic Number Of Oxy
Atoms may not change their atomic numbers or mass numbers except by veryenergetic nuclear reactions. However atoms may gain or lose electrons in ordinary chemical reactions. If an atom has the same number of electrons as protons, it is a neutral atom. If it has a net charge, (more or less electrons than protons) it is an ion.If it has more electrons than protons it has a net negative charge and is known as an anion. If it has fewer electrons than protons it has a net positive charge and is known as a cation. The ionic state may be denoted as a following superscript (e.g. O2-, Fe2+). The common ionic states of a atom are known as its valences.
Because electrons arrange themselves in discreet orbitals about the nucleus and the orbitals repeat in shells, the chemical properties of the elements tend to repeat as the atomic number increases. This periodicity of properties gives rise to the periodic table of the elements.
Atomic Number Of O
The elements H, He, and minor amounts of Li were formed in the original Big Bang. All heavier elements were formed form the primordial H and He by nuclear fusion reactions in stars. The fusion reaction proceeds in steps in stars massive enough to undergo the full sequence. (Our sun is not massive enough to form elements more massive than He by direct fusion and will die when all the H is consumed.) First H is consumed to form He. When the H is consumed, the star collapses until He is 'ignited' to form Be and C. There are many free neutrons in these reactors and nuclei will capture enough neutrons to stabilize themselves. Most of the heavy elements are formed by neutron capture rather than by direct fusion. In the last stage Fe is formed by direct fusion of Si and other light elements. This reactions is rapid and results in an explosion. Our solar system condensed from the remnants of one of these supernova explosions.
Chemical bonds may be either ionic, metallic, covalent or vander Waals (mirror charge), and the bond type preferred by the various elements will determine their geochemical affinity. Ionically bonded elements are termed lithophile and combine with the most abundant element, O, and are enriched in the silicate and oxide minerals (rocks). Metallically bonded elements are termed siderophile and combine with native iron and are enriched in the core. Covalently bonded elements are termed chalcophileand combine with sulfur and are enriched in ore minerals. The atmophile elements form only very weak vander Waals bonds and did not condense in the inner solar system. They are depleted in Earth and enriched in the outer planets.
Here is a full Periodic Table of the Elements.Geological Periodic Table
GEOL 1010 Syllabus